The limitations of manual methods for Health and Safety Management

Health and Safety Management is vital for keeping workplace safe and ensuring productivity. More importantly, effective Environmental, Health and Safety (EHS) management systems enable organisations to meet appropriate health, safety, and environmental standards.
According to the U.S. Bureau of Labour Statistics, employers reported approximately 2.7 billion work-related injury and illnesses in the United States in 2020. Of that massive figure, almost 5,000 of those cases resulted in fatalities. While many of these unfortunate incidents may be tragically unavoidable, there are a significant number of them that can be prevented.
Typical industries such as construction, manufacturing, and pharmaceutical are considered to have higher rates of injuries and fatalities. That is, in comparison to other industries that don’t require strenuous work or exposure to harmful equipment and materials.
With potent health and safety management systems, organisations in these sectors can improve health and safety. Subsequently, this can minimise the number of work-related injuries, diseases and fatalities. As well, it can reduce the financial and overall negative impact on businesses and workers.
Today, many organisations are transforming their manual-based health and safety tasks with flexible EHS software to ensure health and safety of their employees.
Limitations of manual health and safety management
Spreadsheets and paper documents may be used in small routine tasks, but the scope of EHS management is simply too wide to integrate using spreadsheets. The main limitations of using spreadsheets in health and safety departments departments include:
- Human error
A health and safety management system that relies on unautomated tools such as transferring data from paper to spreadsheets is highly prone to errors during the stages of data input and storage. In other words, trivial mistakes will often arise as humans are affected by incompetence and other factors that affect the quality of human-dependent tasks.
- They are not user-friendly
One of the biggest challenges associated with spreadsheet-managed systems is usability. There are countless cells for each metric, posing a serious problem. Moreover, as data expands, so do spreadsheets making it near impossible to keep track of processes.
- Lack of security
Spreadsheets and paper documentation are vulnerable to unauthorised changes and access. A health and safety process usually contains important information about the company’s workplace activities. Hence why spreadsheets may not offer the appropriate level of security to safeguard internal policies.
- Irregular updates
A standard spreadsheet for EHS management is created by several people within an organisation. Typically, the main document is usually uploaded to an internal repository where members of the organisation can review it. This system is unreliable because, sooner or later, some people will have a different version of the same document.
- Scalability
Using spreadsheets or paper-based documentation presents a scale limitation. For instance, your data will grow exponentially as you define more EHS routines and how they can be implemented. A larger spreadsheet or paper folder is more prone to mistakes. Therefore, this makes it difficult to trace specific information and affects the organisation’s ability to evolve processes in alignment with new requirements.
The problems with inefficient health and safety management
The lack of an efficient health and safety management eventually leads to operational inefficiency and creates a hazardous work environment.
In manufacturing, the absence of clear EHS guidelines may expose workers to harmful components, which usually leads to class action lawsuits.
Similarly, construction, and pharmaceutical sites pose great danger to workers due to heavy machinery, damaged and hazardous equipment and so on.
NSC Injury Facts states that the total cost of work injuries was estimated to be $163.9 billion in 2020 alone. This meant that the cost per work was approximately $1,100.

Inefficiently managing health and safety can be extremely expensive and risky for both employers and their employees. For instance, unreliable systems make it difficult to diagnose and document arising issues in the workplace. An organisation that fails to monitor and document its solutions to recurrent EHS problems is doomed to face the same issues in the future.
Why do leaders search for flexible and user-friendly EHS software?
Health and safety management are a standard operating measure in manufacturing, healthcare, engineering, and other organisational industries. Using a digital health and safety system do away with cumbersome and inefficient spreadsheets and offer the following benefits:
1. Optimisation in health and safety procedures
Flexible, user-friendly EHS software enables team members to maintain a central framework of safety processes. This ensures safer and more engaged workplace.
2. Better control
EHS software ensures compliance and accurate collection, tracking, and generating health and safety data. A reliable EHS software enhances transparency and accountability by enabling routine inspection, error captures, and log monitoring. This further streamlines the process of system audits and submission of complaints and other issues.
3. Supporting teamwork
Teams that use decentralised resources can collaborate better and complete tasks quickly together. An effective EHS software can define roles and organisational hierarchy in EHS management. As a result, EHS leaders, legal inspectors, and EHS professionals can master collaboration by using a single point to carry out their duties and manage their interactions.
4. Regulatory compliance
Beyond ensuring a safe work environment, EHS software is important when liaising with legal authorities as they demonstrate clear safety measures within an organisation. Simply, this facilitates a company’s ability to ensure compliance with industry regulations.
Key features of EHSwise software
The features and capabilities of any quality or EHS system have a major impact on the outcomes of health and safety in an organisation.
EHSwise’s flexible platform for Health, Safety and Environment processes is comprised of many unique features that empower health and safety leaders to achieve safety excellence.
Take a look at some of EHSwise features below:
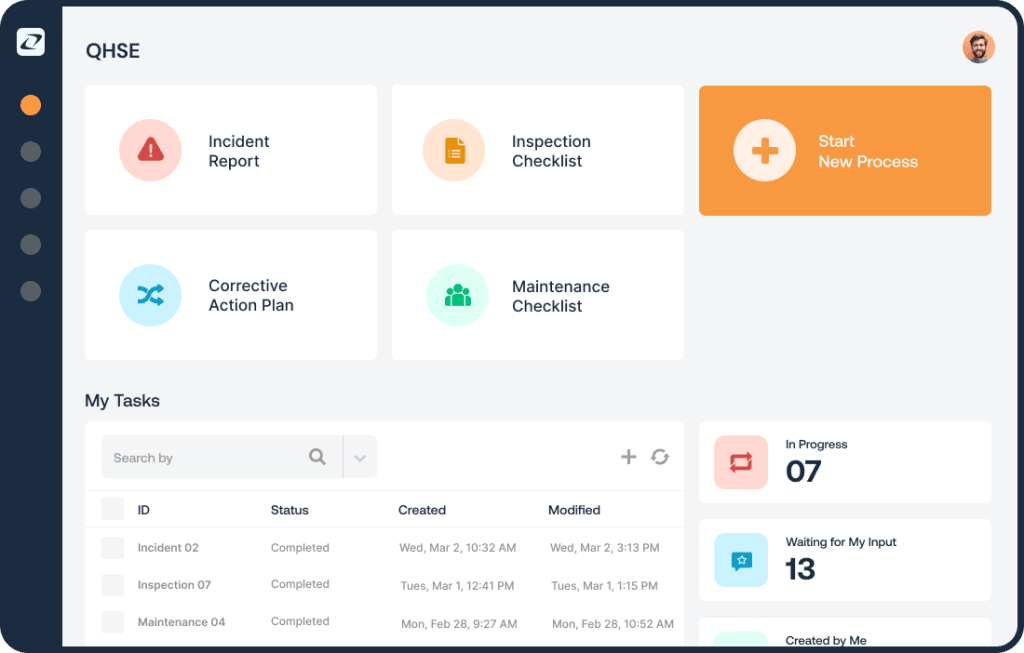
- All-in-one platform
EHSwise unified platform provides a single point of interaction for all the personnel involved in health and safety management. Moreover, it offers a central point for coordinating and aligning an organisation’s practices to observe regulatory safety requirements and quality control.
- Offline & mobile capabilities
This is particularly exciting for teams that work from different places such as companies who operate in remote areas or that have multi-functional teams across the globe. With EHSwise, the platform is greatly accessible offline and on registered mobile devices, and this way, everyone is plugged in from wherever they are.
- A friendly user interface
Beyond an application’s core functionality, its visual attributes play a role in its effectiveness. The EHSwise platform is intuitive, simple, responsive and consistent with its main functions. Because of this, companies can ensure a positive end-user experience for their employees.
- Dynamic dashboards
The platform lets you define user data visibility across different dashboards. This way, everyone has access to the same dashboard but can only view what they are authorised to as per the system specifications. Kianda dashboards offer deep insight into process overview, providing real-time analytics and easy reporting on-the-go.
- Data integration
The EHSwise platform seamlessly integrates with numerous data connectors, creating a central resource for quality, health and safety management processes. In other words, Kianda creates a single source of truth for data by enabling teams to easily connect to legacy and other industry and IT systems such as Procore, Autodesk, SAP, SharePoint, SalesForce, Google Drive, SQL Server, Oracle, and many others.
How does EHSwise help Industry leaders?
Industry leaders use the EHSwise platform to gain full control over health and safety and deliver high-standards of health and safety. See how a world leader in the highly specialised fields of dredging, land reclamation, marine infrastructure, offshore energy and environmental remediation company DEME built an in-house QHSE platform here.
EHSwise unified platform ensures the system is all-inclusive. In short, this guides the designation of roles as defined by the platform, which enhances accountability and cohesion throughout an organisation. As a result, corporate leaders can drive the company to achieve safer workplace since everyone knows their duties.
With mobile and offline capabilities, there is no barrier to the uptake of health and safety management. A significant part of corporate leadership lies in the ability to equip personnel with the right tools for the job supported by technology.
Furthermore, industry leaders can access a simpler, more intuitive platform that aids decision-making. Data integration makes it easier to formulate KPIs and implement EHS processes based on the existing architecture.
In conclusion, health and safety management with a modern, user-friendly EHS software separate average companies from those that stay ahead.


